

 News
News Industry News
Industry NewsIn 1889, German scientists Elster and Gelthier discovered the existence of negative air ions.
In 1902, Chamath and others also confirmed the biological significance of negative air ions.
Under natural conditions, air molecules are neutral and uncharged. However, under the influence of external factors such as ultraviolet light, trace element radiation, and lightning, air becomes ionized, and molecules lose some of their outermost electrons orbiting the nucleus, becoming free electrons. These free electrons combine with neutral molecules in the air to form negatively charged negative ions.
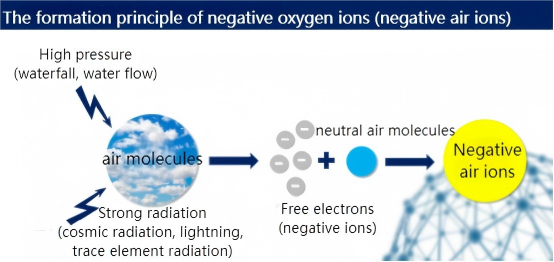
When we walk by the sea, stop by a lake, or enjoy the fresh air of a forest, fountain, or waterfall, we feel relaxed and happy because the air is rich in negative ions.
When negative ions (negative oxygen ions) in the air come into contact with bacteria, mold, viruses, etc., the excess electrons they carry can disrupt their molecular protein structures, leading to structural changes (protein polarity reversal) or energy transfer, which can kill microorganisms such as bacteria and viruses. This is why negative ions can disinfect and sterilize. Although countless air purifiers marketed as "negative oxygen ions" are available, many produce high concentrations of ozone during operation, causing discomfort, impairing the immune system, and in severe cases, causing cancer.
CCTV has also reported on this issue, stating that inspections of several air purifiers from different brands revealed that "ozone concentrations released by them exceeded the standard by more than 10 times, posing a threat to the human respiratory system. When ozone concentrations reach 200 micrograms per cubic meter, they can damage the central nervous system and cause headaches, chest pain, and decreased thinking ability."
A certain ozone concentration is crucial for ensuring disinfection effectiveness, energy conservation, and pollution prevention. Therefore, when using a negative ion disinfector, it is important to monitor the ozone concentration and ensure it is within the standard range. This requires the use of an ozone sensor for monitoring.
Electrochemical sensors are generally used. Electrochemical modules are the best choice for ease of use, saving development time.

TE03-O3 electrochemical module
Tensensor's TE03-O3 module uses electrochemical principles to detect ozone gas in the air. It can detect ozone concentrations from 0 to 10 ppm with a resolution of 0.1 ppm, offering excellent selectivity and stability. It also features a built-in temperature sensor for temperature compensation and a digital output for ease of use.